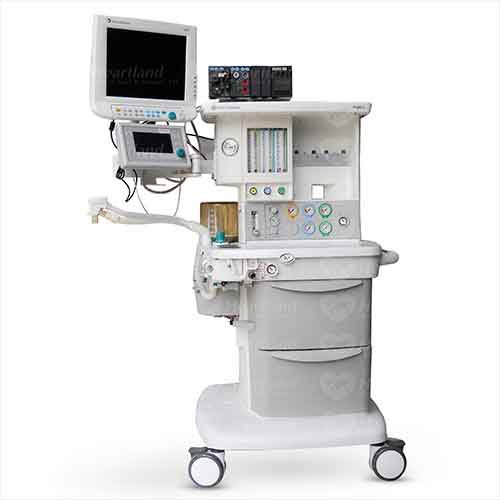
GE Ohmeda S/5 Aespire
The S/5 Aespire provides proven performance with the 7100 Ventilator and Datex-Ohmeda monitoring technology in a system designed to be affordable.
The modern, efficient and compact design of the S/5 Aespire addresses many ergonomic challenges including unique cable management solutions, and a substantial work surface. Optional integrated features include auxiliary O2, suction control and multiple S/5 monitoring integration kits. To make clinical needs less cumbersome, the workstation provides ample storage space with two large drawers. The LED light strip that illuminates bi-level work surface controls that are conveniently located to assist clinicians while sitting or standing. The 7100 Ventilator on the S/5 Aespire has been specifically engineered to enable ventilation for a wide range of patients, from pediatric to adult cases. This medical equipment represents an ideal solution for clinicians seeking a high performance ventilator at an attractive price.
S/5 Aespire represents the introduction of the Advanced Breathing System (ABS). This integrated breathing system offers:
- Easy on/off capability, facilitating easier cleaning and reduced maintenance time.
- Ease of disassembly requiring no tools and fully autoclavable cleaning .
- Complete detection of the ABS utilizing integrated electronics.
- Available with 7900 Ventilator and optional SIMV and PSV Pro packages.
Features
- Enhanced monitor integration capability with our Datex-Ohmeda Anesthesia Monitor and Compact Anesthesia monitor
- Lightweight and compact for improved maneuverability
- Optional integrated auxiliary O2 flowmeter and suction control
Advanced Breathing System (ABS)
- One step bag/vent switch turns the ventilator on/off
- Minimal number of parts and tube connections significantly reduces the potential for leaks and misconnections
- Ease of disassembly (no tools)
- Fully autoclavable and latex-free
7100 or 7900 Ventilator
- Volume and Pressure modes with electronic PEEP
- Exhaled volume, airway pressure and inspired oxygen monitoring capabilities
- Direct access to ventilator parameter settings
- Smart alarms direct user to specific problems and affected parameters
- Pressure bar graph for visual reference on a breath-by-breath basis (optional pressure waveform available)
Improved Low Flow/Reduced Life Cycle Costs
- Fresh gas flow compensation – automatically (available with tidal volume compensation option)
- Minimum O2 flow of 50 mL
- Dual air flow tubes standard for higher resolution of low flows
Dimensions
- Height: 134.5 cm/52.9 in
- Width: 72 cm/28.3 in
- Depth: 73 cm/28.7 in
- Weight: Approximately 108 kg/238 lb
Top Shelf
- Weight limit: 34 kg/75 lb
- Width: 66 cm/26 in
- Depth: 40 cm/15.75 in
Work Surface
- Height: 81.7 cm/32.2 in
- Size: 2160 cm-2/334 in-2
DIN Rail
- Side of machine: 34.5 cm/13.6 in
Drawers (Internal Dimensions)
- Height: 17.5 cm/6.9 in
- Width: 33 cm/13 in
- Depth: 26.5 cm/10.4 in
Absorber Bag Arm (Optional)
- Arm length: 30.5 cm/12 in
- Bag arm height (adjustable): 87 cm (34.3 i) to 104 cm (40.9 in)
Casters
- Diameter: 12.5 cm/5 in
- Brakes: Individual locking front casters
Current Leakage
- 100/120 V: < 300μA
- Power
- Power input: 100-120 Vac, 50/60 Hz
- Power cord: Length: 5 m/16.4 ft
- Rating: 15A @ 120 Vac
Inlet/Outlet Module (120V)
- System circuit breakers: 15A
- Outlets (optional): 4 outlets on back, 3-2A, 1-3A individual breakers, optional isolation transformer
Auxiliary Common Gas Outlet
- Connector: ISO 22 mm OD and 15 mm ID
Gas Supply
- Pipeline input range: 240 kPa to 600 kPa/35 psig to 88 psig
- Pipeline connections: DISS-male, DISS-female, DIN 13252, AS4059, S90-116 or NIST (ISO 5359). All fittings available for O2, N2O, and Air, and include pipeline filter and check valve.
- Cylinder input: Pin indexed in keeping with CGA-V-1 or DIN (nut and gland); contains input filter and check valve. Note: Maximum 3 cylinders; two inboard mounted, one outboard mounted.
- Primary regulator diaphragm minimum burst pressure: 2758 kPa/400 psig
- Primary regulator nominal output: < 338 kPa/49 psig Pin indexed cylinder connections
- < 407 kPa/59 psig DIN cylinder connections
System Operation
- Temperature: 10° to 40°C/50° to 104°F
- Humidity: 15 to 95% relative humidity (non-condensing) per IEC 68-2-3
- Altitude: -440 to 3565 m/500 to 800 mmHg
System Storage
- Temperature: -15° to 50°C/-5° to 122°F
- Humidity: 10 to 95% relative humidity (including condensing) per IEC 68-2-3
- Altitude: -440 to 5860 m/375 to 800 mmHg
- Oxygen cell storage:
- -15° to 50°C/5° to 122°F
- 10 to 95% relative humidity
- 500 to 800 mmHg
Electromagnetic Compatibility
- Immunity: Complies with all requirements of EN 60601-1-2
- Emissions: CISPR 11 group 1 class B
- Approvals:
- UL 2601-1
- CSA C22.2 #601.1
- EN/IEC 60601-1
- CE 0197
Your Heartland Medical Support Team
Heartland Medical’s support professionals are committed to enhancing patient care while protecting your investment.
Dedicated field service and in-house technical support experts are here to help 24 hours a day, 7 days a week!
Please Contact Heartland Medical Sales & Services today for additional information and to get a free quote!
Condition: Remarketed
Defibrillators:
Department:
Electrosurgical Units:
Equipment Type: Anesthesia Machine
Flow Control: Analog|Low-Flow
Modes of Ventilation Available: PCV | PSV | SIMV | VCV
Monitoring Options Available: VPO
Offers:
Patient Monitors:
Production Status: Current Production Model
User Manual:
Vaporization: Traditional